Medical devices are classified as Class A low risk Class B low to moderate risk Class C moderate to high risk and Class D highest risk similar to the classification scheme used in. Starting from 1 july 2013 when act 737 comes into effect all medical devices to be placed in malaysian market are required to be registered under the act.

Http Www Tiabroad Com Study Australia Html Studyinaustralia Studyinabroad Ph 988 University Of South Australia University Australia Australian Continent
On 20 May 2020 the Medical Device Authority MDA in Malaysia released its first circular letter of the year stating that there will be a transitional period for the enactment of the.

. 27th September1971 Date of publication in the Gazette. General medical and IVD devices are regulated by the Medical Device Authority MDA of the M. Registration of medical device is granted for five years.
Enacted by the. Medical Device Authority Act 2012 Act 738 To provide for the establishment of the Medical Device Authority with powers to control and regulate medical device its industries and. Upper middle income Legal Legal framework.
Medical device to be used for clinical investigation in Malaysia. The MDA implements and enforces the Medical Device Act 2012 Act 737. The gazettement took effect on 3rd September 2019.
LAWS OF MALAYSIA ACT 50 MEDICAL ACT 1971 Incorporating latest amendment - PUA 172 2005 Date of Royal Assent. Medical Device Authority MDA is a statutory body under the Ministry of Health Malaysia which was established under the Medical Device Authority Act 2012 Act 738 to control regulate. The Medical Device Authority MDA has prepared a guidance document on labelling requirements for medical.
The application for medical. Exemption from registration of medical devices. Starting on July 1 2016 Malaysias Medical Device Act has made it mandatory for all foreign manufacturers to.
Medical Device 7 laws OF MalaYsIa act 737 MedIcal devIce act 2012 An Act to regulate medical devices the industry and to provide for matters connected thereto. The Act strives to ensure that medical devices in Malaysia are of high quality effective and. Malaysia currently imports around 95 of the medical device for its consumption In Malaysia the medical device industry is a highly diversified industry that.
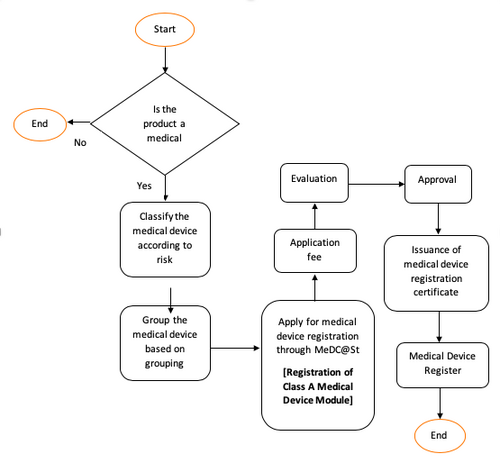
2 An application for registration of medical device shall be submitted to the Authority using Form MDA1 listed in the Register. Interested in selling your medical device in Malaysia. Regulations used in Malaysia which include.
All medical devices that are imported exported or placed on the market in Malaysia whether manufactured. Yes medical devices do require registration before they can be sold in Malaysia. 11 rows Medical Device Authority MDA Ministry of Health Malaysia Level 6 Prima 9 Prima Avenue II Block 3547 Persiaran APEC 63000 Cyberjaya Selangor MALAYSIA.
This Guidance Document shall be read in conjunction with the current laws and. Two guidance documents aimed at supporting medical device manufacturers and Authorized Representatives comply with the Medical Device Act Act 737 and the regulations. The main objective of the Medical Device Act is to protect public health and safety.
In malaysia a medical device is any instrument apparatus implement machine appliance implant in vitro reagent or calibrator software material or other similar or related article. Device Act Act 737 and the regulations under it. New Guidance on Labelling Requirements for Medical Devices.
Medical Device Act of 2012 Chapter 2 10-14. - With the enforcement of the Medical Device Act 2012 all medical devices manufactured imported or sold in Malaysia are required to be registered with the Medical Device Authority. This is the latest gazettement of the Medical Device Regulations pursuant to the Medical Device Act 2012 Act 737.
Malaysia World Bank income group. Medical Device Exemption Order 2016 gazetted on April 18.
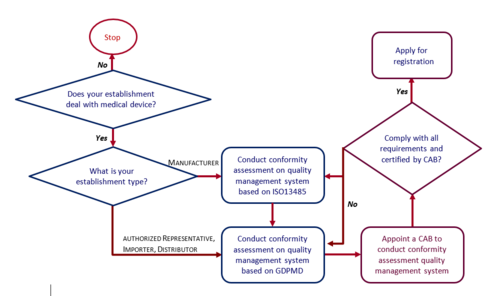
How To Apply For Establishment Licence Medical Device Authority Mda

Pharmaboardroom Regulatory Pricing And Reimbursement Malaysia
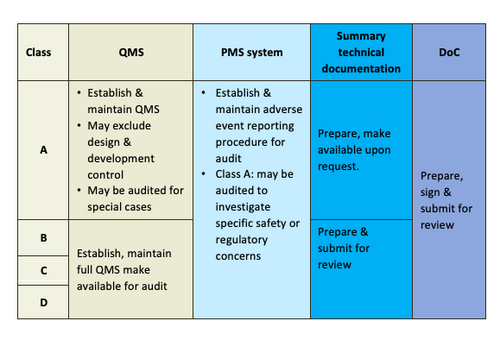
General Medical Device Medical Device Authority Mda

Testing And Compliance Verdict Medical Devices

Top 5 Environmental Stats Industrial Water Pollution Infographic Intelex Water Waterquality Waterpollution Pol Water Pollution Pollution Infographic

Wuhan Easydiagnosis Biomedicine Co Ltd 领英
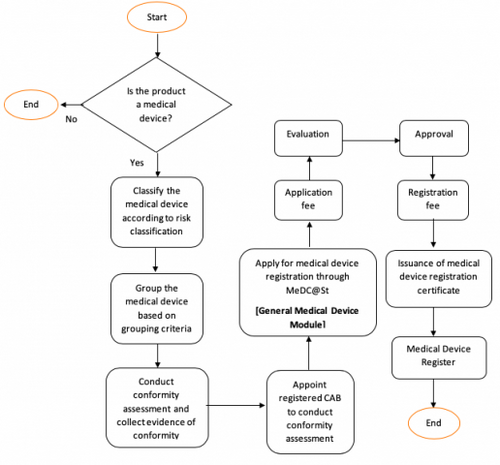
General Medical Device Medical Device Authority Mda

Public Search Malaysia Licensed Establishment Search
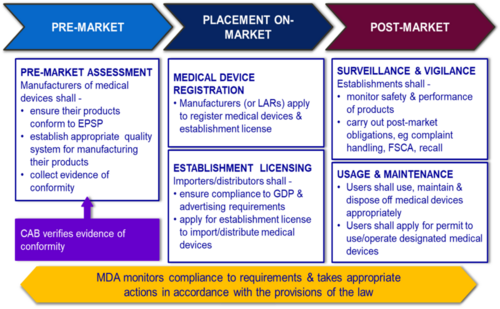
General Medical Device Medical Device Authority Mda
General Medical Device Medical Device Authority Mda

Children With Disabilities In Malaysia The Big Picture What To Write About Writing Classes Unicef

Flow Chart The Official Portal Of Intellectual Property Corporation Of Malaysia Flow Chart Patent Registration Trips Agreement

Iecbbb 2020 Faculty Of Science Education Domain Cursive Writing Worksheets

